Pyrolysis fuel oil (PFO) is a petroleum residue oil obtained from naphtha cracking centers in the petroleum refining process. It has received much attention due to such advantages as its high aromaticity, low sulfur content, low toluene-insoluble content, and low cost [1-4]. PFO is especially suitable for the manufacture of pitch, which is a precursor of several carbon materials [5-7]. PFO can be converted into pitch through heat reaction treatment. The conditions of the PFO heat reaction play a very important role, as they can influence the chemical structures of the produced pitch [8-10]. Currently, researchers are studying pitch extraction from PFO using various methods and conditions.
Jung et al. [11] studied the reforming of PFO by electron beam (EB) radiation for pitch production. PFO containing AlCl3 as a catalyst was heated to 270℃ and subsequently irradiated by EB. As a result, the pitches obtained by EB treatment had decreased nitrogen, hydrogen, and sulfur contents; moreover, the aromaticity, quinoline-insoluble values, toluene-insoluble values, softening point, and molecular weight increased. EB treatment for the conversion of PFO into pitch was found to be useful in preparing aromatic pitches.
Jung et al. [12] investigated the effect of ultraviolet (UV) irradiation on the production of pitch from PFO. As-received PFO was treated with heat and then irradiated by UV. The UV irradiation decreased the hydrogen content and increased the aromatic carbon compounds and the softening point of the obtained pitch. Therefore, UV irradiation was also found to be an effective method for the preparation of pitch from PFO.
In this study, PFO was pretreated by oxidation with H2O2 to increase the carbon yield, and then thermally treated for various times to increase the carbon yield. The obtained pitches were characterized by Fourier transform infrared spectroscopy (FT-IR), H nuclear magnetic resonance (NMR), carbon yield and electrical conductivity.
PFO, supplied from GS Caltex Corporation (Jeonju, Korea), was pretreated by oxidation using H2O2 injection at 110℃ to distil and remove volatiles. Oxidation-pretreated samples were then subjected to different heat treatment times (HTT) of 1, 3, 5, 7, and 10 h at 360℃ under N2 flow. The FT-IR spectra were obtained using a Bruker Tensor 37 (Billerica, MA, USA) to determine changes in the functional groups caused by each treatment. All samples were prepared as thin films, and mixed with KBr at a sample/KBr ratio of 1:100 (w/w). The spectra obtained were the results of 30 scans at a spectrophotometer resolution of 8 cm−1. The chemical structures of the obtained pitches were identified by 1H NMR spectra (CXP-40, Bruker), measured at a resonance frequency of 38.27 MHz on a spectrometer equipped with a high-temperature probe. Electrical resistivity analysis was measured using a 4-point probe device by Mitsubishi Chemical Corp. (Tokyo, Japan).
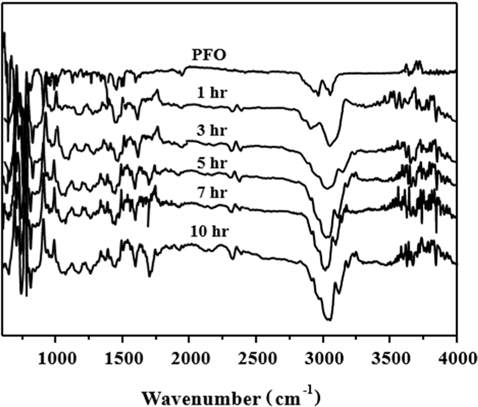
FT-IR analysis was carried out in the 400–4000 cm−1 range to identify changes to the functional groups of the PFO-derived pitches obtained from different HTTs after oxidation pretreatment. As observed in Fig. 1, the spectrum of the PFO raw material shows the adsorption peak for the aromatic C–H out of plane bending bonds at 700–900 cm−1, the aromatic C–H bonds at 1600 and 3042 cm−1, and the stretching C–H bond at 2910 cm−1. However, in the case of the PFO-derived pitches, the intensities of the aromatic C–H bonds at 1600 and 3042 cm−1 gradually increase, and the intensities of the stretching C–H bond at 2910 cm−1 decreases with an increase in HTT. These results indicate that the degree of polymerization of the aromatic rings in the PFO-derived pitch considerably increases as the HTT increases.
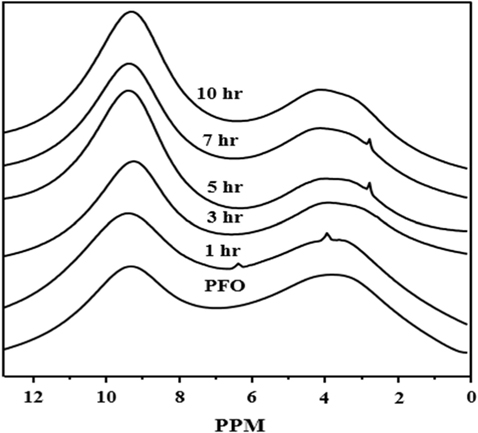
Fig. 2 shows the 1H NMR spectroscopy for PFO-derived pitches. In Fig. 2, all the samples show two separate low portion bands in the range of 1–12 ppm, with high intensity at 7–11 ppm, related to the aromatic portion, and low intensity at 2–6 ppm, attributed to the aliphatic methyl hydrogen portion. The intensity of the aromatic portion of the PFO-derived pitch strongly and sharply increases compared to that of the aliphatic methyl hydrogen portion as the HTT increases. Therefore, this indicates that the aromaticity of the PFO-derived pitch increases with an increase in HTT.
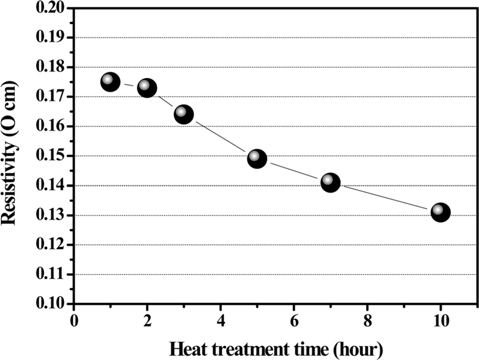
Fig. 3 shows the electrical resistivity of PFO-derived pitches obtained according to HTT after oxidation pretreatment. The resistivity of the obtained samples decreases monotonously with an increase in HTT. In addition, the carbon yields increase with an increase in HTT. These results indicate that an increase of the aromatic portion in the PFO-derived pitch influences the decrease in electrical resistivity.
In conclusion, we studied PFO-derived pitches prepared with different HTT after oxidation pretreatment by H2O2. The obtained pitches were evaluated by FT-IR, NMR, and electrical resistivity. In the FT-IR analysis, the structural conversion of the PFO-derived pitches showed an increase in the intensities of the aromatic C–H bonds at 1600 and 3042 cm−1 and a decrease in the intensity of the stretching C–H bond at 2910 cm−1 as HTT increased. Furthermore, NMR analysis showed that the intensity of the aromatic portion (7–11 ppm) of the PFO-derived pitch increased with an increase in HTT.