Rapid urbanization has caused a number of environmental problems, including heavy metal-containing wastewater, air pollutants from vehicles and factories, and global warming due to the increasing fossil fuel consumption; which increasingly threaten the quality of human life [1-8]. In particular, indoor air pollution, which is known to cause “sick building syndrome,” has attracted significant attention because the time urban residents spend indoors has become an increasing portion of their total activities. Construction materials, interior pieces and wallpaper, and furniture used during the construction of houses and buildings, contain a range of volatile organic compounds, including benzene, toluene, and formaldehyde. In addition, other air pollutants, such as nitrogen oxide, carbon monoxide, and asbestos, are also emitted indoors. Exposure to these pollutants in closed spaces can cause headache, dyspnea, atopic dermatitis, urticaria, heart disease, and cancer [9-11].
Formaldehyde is a representative indoor air pollutant among those responsible for sick building syndrome. Efforts have been made to remove formaldehyde effectively using photocatalytic oxidation [12], catalytic oxidation [13], and biodegradation [14]. Nitrogen oxides are not only indoor air pollutants, but are also the main industrial gaseous wastes emitted from coal combustion processes as well as urban air pollutants emitted from vehicles. The most popular technology for removing nitrogen oxides is selective catalytic reduction [15]. This method, however, cannot be used at room temperature and consumes a large amount of energy. This is why there is still demand for a simpler, more effective, and more economical pollutant removal technology.
Activated carbon is used widely for the adsorption of noxious gases and heavy metals, as well as for wastewater treatment because of its large adsorption capacity, large surface area, and high porosity [16-25]. On the other hand, the price of commercial activated carbon produced from bituminous coal, coconut shell, wood, or peat is relatively high because of the costly raw materials and expensive activation process [26].
One of the most important global problems is climate change due to global warming. The application of biomass-derived char as an adsorbent is beneficial not only to the removal of pollutants but also to the reduction of carbon emissions. In particular, use of the byproduct biochar (obtained from fast pyrolysis of biomass aimed at the production of bio-oil) would provide an additional economic advantage [27-39].
Biochar is obtained from the pyrolysis of a wide range of biomass materials, such as woody materials and agricultural wastes (olive husk, corncob, tea waste, green waste, etc.) [30,40-44]. Having a microporous structure and high C content, biochar can be used in a range of applications [45]. On the other hand, it has an important drawback: its specific surface area, and therefore its adsorption capacity, is smaller than that of activated carbon. Various activation treatment methods have been proposed to enhance the adsorption capacity of biochar [16,46].
There are approximately 10,000 different species of seaweeds (marine macroalgae), which is more than the number of all land species providing useful biomass. Seaweeds are abundant in coastal regions worldwide. Their life cycle is short; hence the rate of reproduction is high. They are inexpensive and easy to handle. The advantages of seaweeds make them potentially important energy resources [47,48]. Furthermore, they have high selectivity toward the removal of various heavy metal ions, such as gold, cadmium, copper, and zinc (they have exceptional metal binding capacity), making them potential biosorbent materials [49,50]. Seaweed-derived biochar has recently been used for the removal of copper ions in wastewater [46,47,51-53]. To the best of the authors’ knowledge, however, seaweed biochar has never been used for the removal of air pollutants, such as formaldehyde and nitrogen oxide.
In this study, biochar produced from the fast pyrolysis of a representative seaweed species,
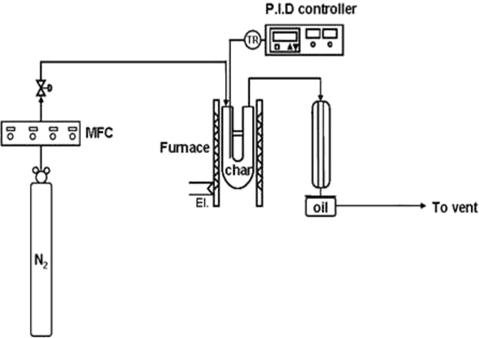
The char produced from fast pyrolysis was activated with steam, KOH, and ammonia to improve its adsorption characteristics. For steam activation, a gas mixture of 80% N2 and 20% H2O was put through a reactor containing PC at the rate of 50 mL/min. The reactor temperature was increased at 5℃/min to 500℃, and then maintained at that temperature for 1 h. This steam-activated char is referred to in this paper as PCW.
For KOH activation, a mixture of KOH and char (mass ratio of 1:1) was dried on a hot plate; then dried further in a 110℃ oven for 24 h. The dried char sample was pulverized, placed into a reactor, and calcined for 1 h at 700℃ under flowing nitrogen (50 mL/min). After washing out the residual K+ ions with distilled water, the char sample was neutralized using a 5 M HCl solution and dried. The char activated this way is referred to in this paper as PCK.
Ammonia activation began by treating PC in a reactor for 2 h with ammonia gas (50 mL/min) at 350℃. The residual ammonia on the char surface was removed using a flow of 50-mL/min N2 gas for 1 h. The char activated this manner is referred to in this paper as PCA.
PCK was also ammonia-treated in the same manner. The resulting double-activated char is referred to in this paper as PCK + PCA.
2.2. Characterization of the char
Nitrogen adsorption/desorption of the char samples pretreated in a vacuum at 200℃ was measured at 77 K using a Sorptomatic (Thermo Fisher Scientific, Waltham, MA, USA). The specific surface area and total pore volume of the char was determined from the adsorption-desorption isotherms using the Brunauer-Emmett-Teller method [56].
The metal species contained in the char samples were analyzed by inductively coupled plasma atomic emission spectroscopy (ICP-AES, Optima-4300; Perkin Elmer, Waltham, MA, USA).
X-ray photoelectron spectroscopy (XPS) was performed using an AXIS-NOVA (Kratos Inc., Kyoto, Japan). A monochromatic Al Kα (1486.6 eV) X-ray source and 40 eV analyzer pass energy were used in ultra-high vacuum (5.2 × 10-9 Torr).
The thermogravimetric characteristics of the char was examined by thermogravimetric analysis (TGA; Pyris 1 TGA, Perkin Elmer) under a 50-mL/min flow of N2 while the temperature was increased from room temperature to 900℃ at a rate of 30℃/min.
2.3. Adsorption of formaldehyde
Adsorption experiments were carried out according to the procedure suggested in previous studies [16,51,55]. A 10-liter aluminum bag was flushed using N2 gas and vacuumed. Formaldehyde (100 ppm) was inserted into the bag together with N2 gas to adjust the formaldehyde concentration to 1 ppm. Subsequently, 0.07 g of activated char was placed in the bag, which was then placed in a temperature-controlled incubator maintained at 30℃. To enhance the gas-char mixing, the sample bag was stirred in a shaking incubator.
The amount of formaldehyde adsorbed was measured at 0, 10, 40, and 80 min using a formaldehyde analyzer (4000 Series; Woori System, Seoul, Korea).
The NO removal experiments were performed using a fixed bed reactor. The reactant gas composition was set to NO 1000 ppm, NH3 1000 ppm, and O2 5%, with the balance being N2 gas. The total gas flow-rate was 100 ml/min. The amount of catalyst used for each experiment was 0.3 g, with a W/F (char sample weight/feed flow) ratio of 3 g·min/l (S.V.≒10,000 h−1). Commercial activated carbon developed for air cleaning and odor removal was purchased (Kaya Activated Carbon Inc., Seoul, Korea) for comparison with the activated char used in this study.
The reaction temperature was between 50℃ and 300℃ at 50℃intervals. A constant temperature was maintained for 30 min at each temperature. The NOx concentration was determined using a NOx analyzer (42i HL; Thermo Fisher Scientific).
3.1. Characteristics of the P. tenera char
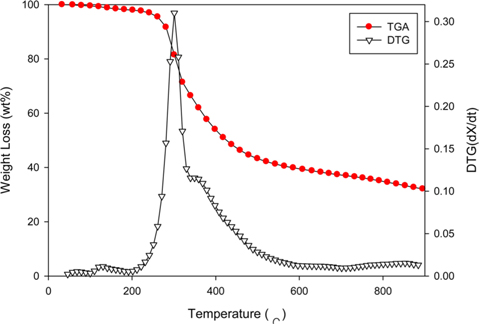
Fig. 2 shows the TGA/differential thermogravimetric analysis of the dried PC. The char mass began to decrease rapidly at approximately 250℃ and the reduction rate slowed down at approximately 500℃. The pyrolysis reactions took place over the temperature range 250℃–500℃. The mass reduction at approximately 100℃ was attributed to evaporation of moisture. After most of the gas and moisture were removed at about 400℃, the mass of the remaining solid decreased gradually. Therefore, 500℃ was chosen as the pyrolysis temperature at which to produce PC.
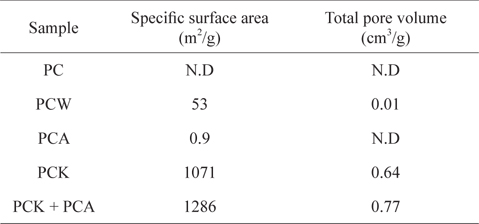
Table 1 lists the specific surface areas and total pore volumes of the kinds of char used in this study. The specific surface area of PC produced at 500℃ was below the detection limit. The steam treatment (PCW) increased the specific surface area to 53 m2/g, whereas the increase in specific surface area by ammonia treatment (PCK) was marginal (0.9 m2/g). The KOH treatment (PCK) led to a huge increase in the specific surface area to 1071 m2/g, which was attributed to the removal of considerable ash content (Table 2), resulting in the opening and development of pores [54,55,57]. When PCK was treated further with ammonia (PCK + PCA), the specific surface area was increased further to 1286 m2/g. The specific surface area increase by treatment with ammonia can be attributed to its etching effects. Mangun et al. [58] reported that the specific surface area increased with increasing duration of ammonia treatment and with temperature.
[Table 1.] Physical properties of Pyropia tenera char
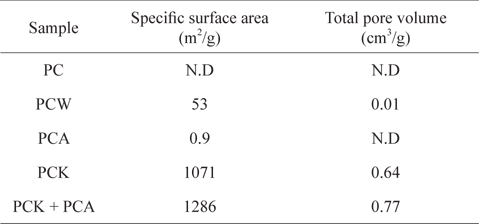
Physical properties of Pyropia tenera char
[Table 2.] Ash content in the kinds of char

Ash content in the kinds of char
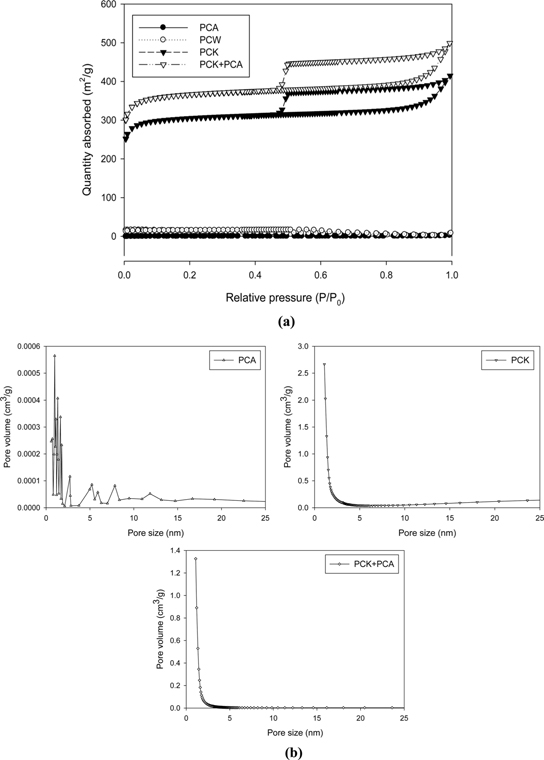
Fig. 3 presents the nitrogen sorption isotherms and the corresponding pore volumes of the char. PCW and PCA exhibited type-I isotherms, whereas PCK and PCK + PCA showed type-IV isotherms with an H1-type hysteresis loop. The pore size shown in Fig. 3b indicates the coexistence of micropores and mesopores in PCK and PCK + PCA.
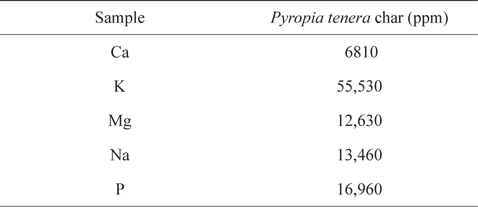
Table 3 lists the ICP elemental analysis results for metals in the PC. The metallic species originated from inorganic matter in the seawater, and accounted for most of the mass of the ash content of seaweed. Among these, Ca, K, and Mg, in particular, enhance the level of formaldehyde adsorption [59].
[Table 3.] Elemental analysis of Pyropia tenera char
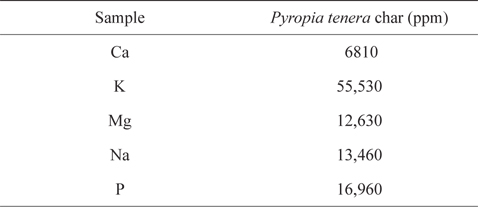
Elemental analysis of Pyropia tenera char
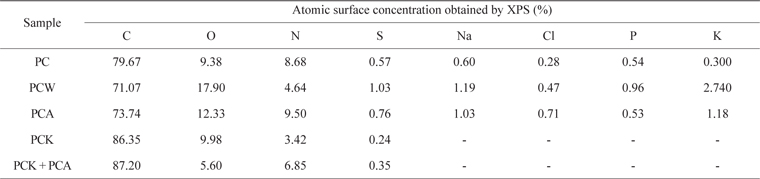
Table 4 presents the XPS results. PCW, PCK, and PCA showed a larger quantity of O than did PC. PCA had a larger quantity of N than did PC due to the ammonia treatment. PCK + PCA also had a larger quantity of N than did PCK for the same reason. Na, Cl, P, and K were removed completely by the KOH treatment (PCK and PCK + PCA), leading to a significantly higher C content [51]. O and N enhanced the adsorption capability of the char by forming oxygen- and nitrogen-containing functional groups, respectively, on the char surface.
[Table 4.] Elemental surface composition of Pyropia tenera char
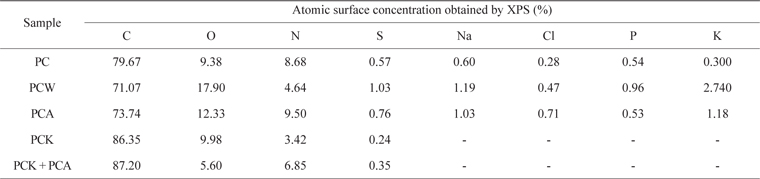
Elemental surface composition of Pyropia tenera char
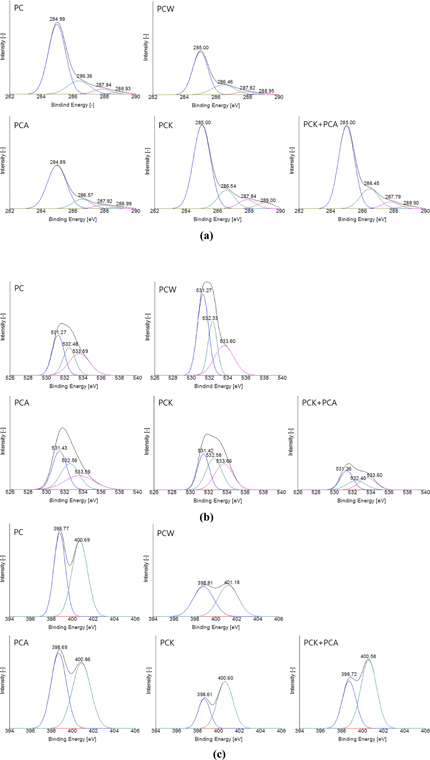
Fig. 4 shows the C1s, O1s, and N1s spectra obtained from XPS analysis of the char. The deconvolution of the XPS spectra enabled the identification and intensity determination of surface functional groups. Fig. 4a presents the C1s spectra. Although the intensity depends on the method of activation, all varieties of the char showed a peak at a binding energy level of 285 eV (±0.5 eV), indicating a C-C bond. Three more peaks were observed in all the samples: C-O, C=O, and COO– peaks appearing at 286 eV (±0.5 eV), 287.9 eV (±0.5 eV), and 288.7 eV (±0.5 eV), respectively [60]. Fig. 4b shows the O1s spectra. All the samples exhibited three common peaks: C=O, C-O, and COO− peaks, appearing at 531.3 eV (±0.5 eV), 532.7 eV (±0.5 eV), and 533.6 eV (±0.5 eV), respectively [60]. Fig. 4c shows the N1s spectra. The common peaks were observed at two binding energy levels: pyridine N and C-N peaks appeared at 398.7 eV (±0.5 eV) and 400.7 eV (±0.5 eV), respectively [58].
3.2. Adsorption of formaldehyde by the P. tenera char
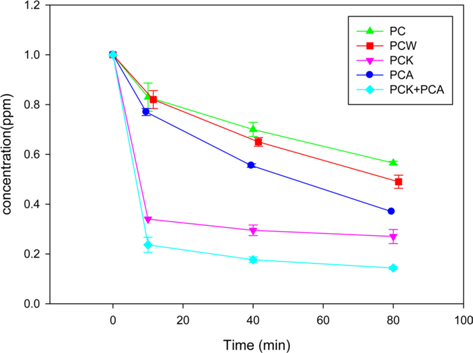
Fig. 5 presents the results of the formaldehyde adsorption experiments using PC, PCW, PCA, PCK, and PCK + PCA. The adsorption rate was highest during the first 10 min, and decreased gradually thereafter. The adsorption of formaldehyde on PCK and PCK + PCA was apparently complete at 40 min. Although PC had the smallest specific surface area and hence the lowest adsorption capability, it showed a formaldehyde removal rate as high as 40% because it contained O- and N-containing functional groups, and a large quantity of metal species. PCW had a larger specific surface area and a smaller quantity of metal species than PC. XPS showed that steam activation increased the quantity of O-containing functional groups on the char surface, resulting in enhanced formaldehyde adsorption. PCA also showed enhanced formaldehyde adsorption ability compared to PC. The adsorption capability of PCA was superior to that of PCW with a larger specific surface area, which was attributed to the large quantity of N-containing functional groups in PCA (see the XPS analysis results). Lee et al. [55] and Yu et al. [61] reported that ammonia treatment of char and mesoporous carbon nitride (MCN) resulted in an increased number of N-containing functional groups and significantly enhanced formaldehyde adsorption ability. The most effective activation method appeared to be KOH activation: PCK exhibited a higher formaldehyde removal rate than did PC, PCW, and PCA. This was attributed to the huge specific surface area (1071 m2/g) and the O- and N-containing functional groups of PCK.
PCK + PCA showed the highest adsorption capability because it had the largest specific surface area (1286 m2/g) and twice the number of N-containing functional groups as PCK.
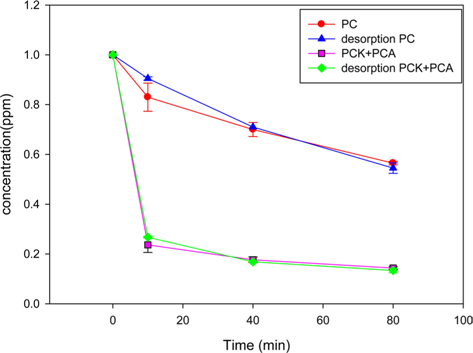
The PC and PCK + PCA were reused after thermal desorption of formaldehyde. Fig. 6 compares the formaldehyde adsorption capabilities of the fresh and recycled chars. The thermal desorption of formaldehyde was conducted under nitrogen gas at 110℃. The reusability of an adsorbent is one of the most important aspects in the economy of adsorption processes. Both PC and PCK + PCA showed little difference between the adsorption rates of fresh and recycled chars, indicating the excellent reusability of both kinds of char.
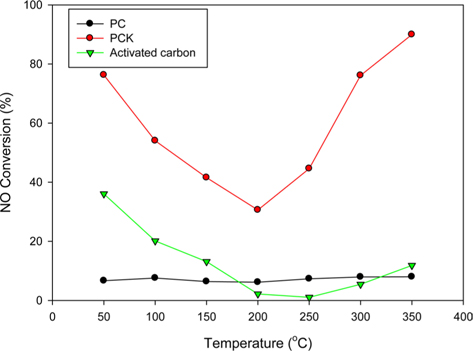
Fig. 7 shows the NO conversions obtained using PC, PCK, and activated carbon at different temperatures. PCK and activated carbon showed typical V-shaped curves. The NO conversion obtained below 150℃–200℃ is generally attributed to adsorption and that obtained at higher temperatures is due to the catalytic removal of NOx [62]. PC showed little NO reduction regardless of the temperature. KOH-treated PCK showed approximately 80% NO adsorption at 50℃, which was even higher than that of commercial activated carbon. The high NO removal capability of PCK was attributed to its large specific surface area and O- and N-containing functional groups. In particular, it showed superior ability for NO reduction compared to commercial activated carbon, highlighting the potential for PCK to be commercialized as an alternative NO-adsorbing material. A more detailed comparison versus activated carbon will be carried out in a future study.
Biochar derived from a seaweed species,