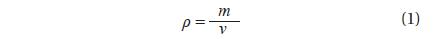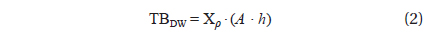A bloom is a developing phenomenon due to the overgrowth of a species in the environment at the expense of others (Cartensen et al. 2007). Such species can be either macroalgae or microalgae. There are several studies related to algal blooms from an international perspective. Algal diversity inside a bloom can be explained by preferences of herbivory activity (Lotze et al. 2000). They can be assigned as red, green, or brown tides, according to the color of the predominant organism, with “green tides” as the most common one. They have been reported in the coast of several European countries (Scanlan et al. 2007), North America (Nelson et al. 2008), and Asia (Liu et al. 2010, Kang et al. 2015). In some places, macroalgae can float freely on the beaches (Piriou et al. 1991, Merceron and Morand 2004).
In Brazil, several blooms have been detected. They are predominantly made up of microalgae with impacts on coastal management, resulting in fish kills and changes to food webs (Freitas et al. 1992). In addition, they can cause human poisoning by direct or indirect ingestion of toxins. Many of these phenomena have been linked to anthropic activities such as discharges from industrial and domestic sewages (Figueiredo et al. 2004).
Blooms of seaweeds are different from the microalgae blooms in at least three aspects. First, they have no direct chemical toxicity. Second, they have a broader range of ecological effects involved. Third, these formations extend for a longer period of time (Hay and Fenical 1988). They may remain in place for years to decades. For example, in the Peel Harvey Estuary in Western Australia, a
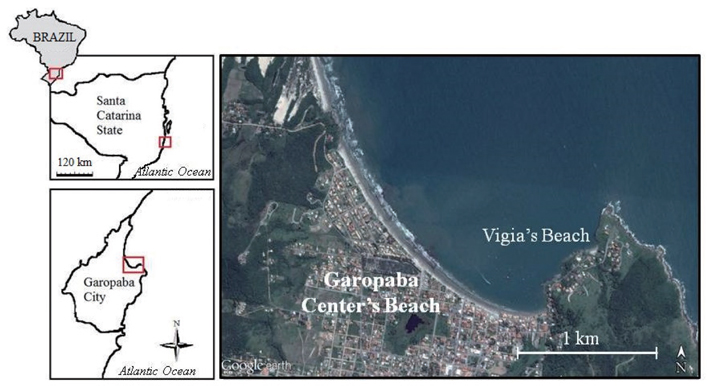
In the summer of 2013-2014, there was a macroalgae bloom forming in the city of Garopaba, Santa Catarina State, Brazil. It aroused the interest of tourists and locals as well as the scientific community. The aim of this study was to characterize and quantify photosynthetic organisms present in this bloom that appeared to be floating in the water column. Moreover, the flora of a neihgbouring rocky shore was evaluated to test the hypothesis that the algae present in this bloom could be part of the local flora. In addition, as algal material was deposited on the sand beach, sampling was also conducted in these areas to compare their compositions to those of the floating species and local rocky shore flora.
Bloom formation was initially detected in the city of Garopaba (total population of 18,000 inhabitants) located in the south of Santa Catarina State, Brazil (Fig. 1). Samples were taken in three different environments including Garopaba Center Beach and a nearby pocket beach called Vigia Beach. First, samples were taken from the floating bloom material (Fig. 2A). Then, algal materials were sampled from the rocky shore near the bloom and from post-beach zone organisms compounding wracks. For the floating organisms, five points were selected randomly, covering an area of 500 m in linear length.
Samplings of the Garopaba Center Beach were performed on 29 January 2014. Floating materials were obtained with a 32,768 cm3 cube built with PVC pipes and surrounded by canvas of approximately 0.5 mm in porosity (Fig. 2B). The cube was positioned into the water, at various depth (0.5 m to 1.20 m). The open side was placed toward the final last wave. It was remained in this position for about 3 s. Then the cube was suspended, bringing the floating organisms from the bloom. The materials were inserted into plastic bags and frozen at −20℃. Algae were collected at 5 points in the bloom. There were 5 replicates at each point, resulting in a total of 25 samples.
Considering the difficulty in acessing the Garopaba Center Beach rocky shores, we performed qualitative collections on the neighbouring rocky shore of Vigia Beach on January 28, 2014. For this purpose, six 900 cm2 quadrats were sampled in the wrack (Fig. 2C & D). Six quadrats were also sampled from the rocky shore. The materials collected on the shore and wrack were also frozen at −20℃.
Samples were thawed, sorted, and identified according to Joly (1967), Cordeiro-Marino (1978), and Pedrini (2011, 2013). Algae were placed in a stove at 40°C until constant weights were achieved. The weight was recorded as dry biomass using an analytical balance (fa2104n model; Bioprecisa, Curitiba, PR, Brazil). After this step, a portion of the material representing of each taxon was deposited at the Herbarium FLOR (Department of Botany herbarium, Florianópolis) at the Federal University of Santa Catarina.
To estimate the algal biomass present in the bloom on the beach of Garopaba center, we used the Eq. (1) where
Software PRIMER 7 was utilized to analyze the differences between the data of dry biomass of the collection points associated with taxonomic composition. Permutational multivariate analysis of variance (PERMANOVA) and similarity percentages analysis (SIMPER) were applied to assess the main species contributing to the phenomenon.
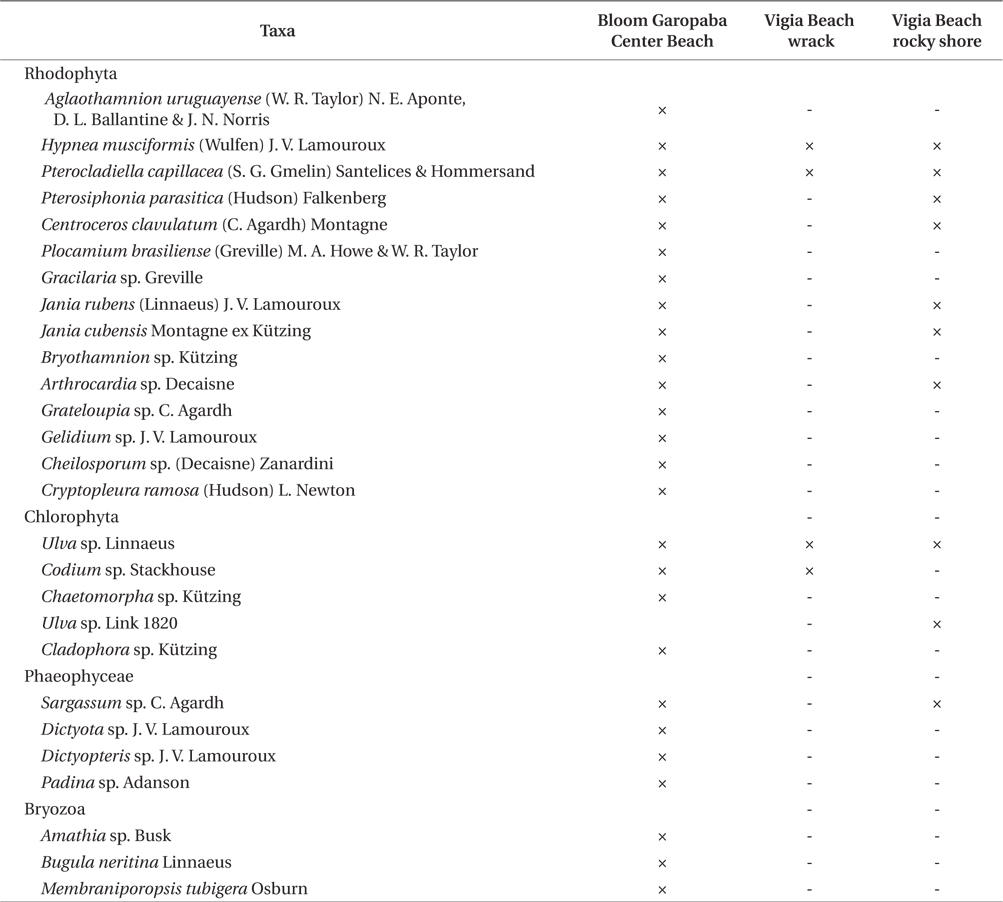
Twenty seven taxa were identified in the bloom, including three species of bryozoans (
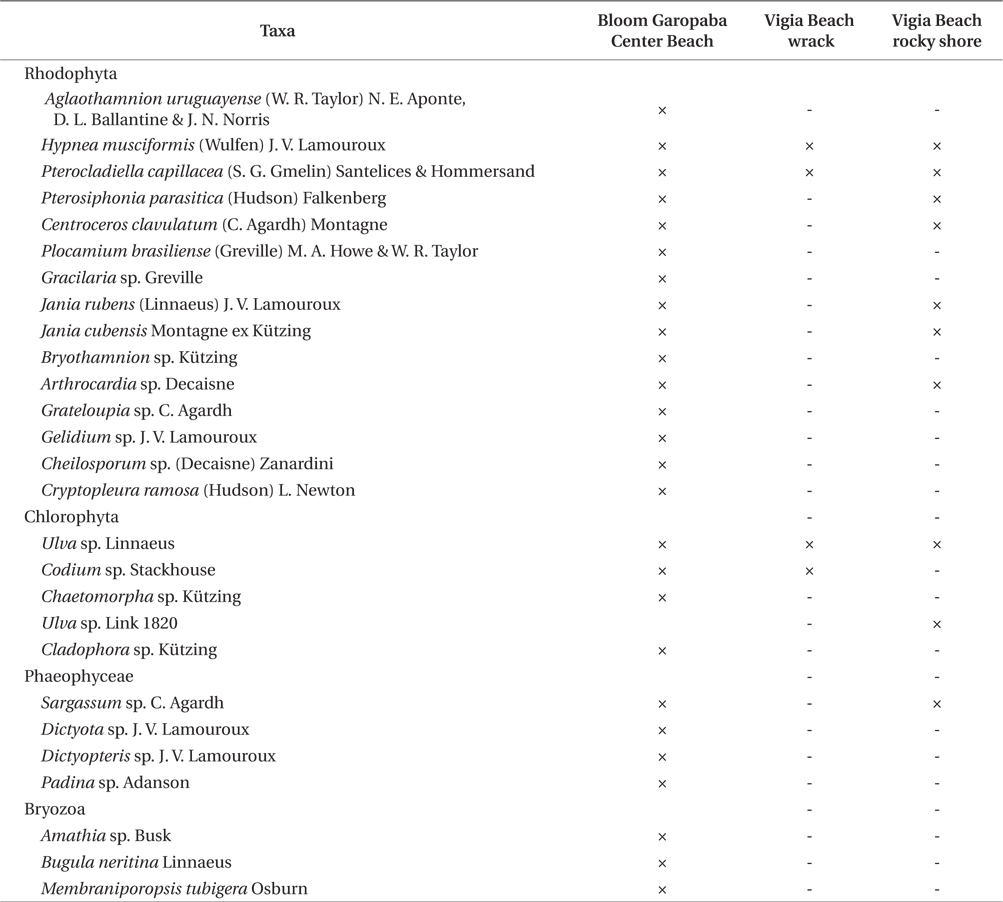
Organisms identified in three environments (bloom floating species at Garopaba Center Beach, wrack material at Vigia Beach, and Vigia Beach rocky shore community of Garopaba, Santa Catarina)
Filamentous red alga
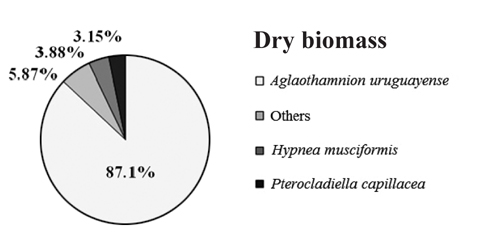
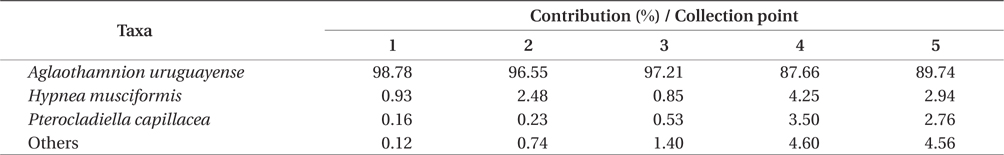
Overall, the biomass found at each of collection points were statistically different (PERMANOVA, Pseudo-F4,20 = 8.538, p = 0.001), indicating heterogeneity in the bloom. Based on SIMPER analysis, the contribution of species in the bloom at each point could be verified. The main species that contributed to the bloom biomass at any of the five points was
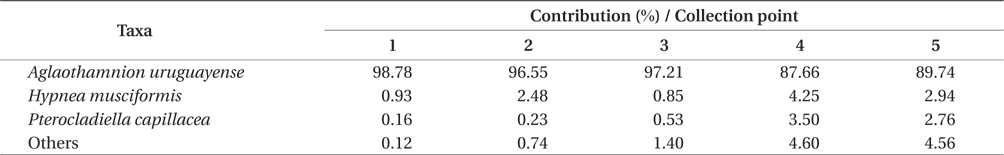
Contribution of different species present to floating algal biomass at Garopaba Center Beach
The macroalgal bloom of Garopaba, Santa Catarina, occurred in the summer season in 2014. It was primarily consisted of
The occurrence of
The constant presence of bryozoans in these samples suggests the importance of understanding other aspects of a forming bloom.
Most macroalgae blooms consists of one or two species, suggesting that they are more sensitive to excess nutrients than other macroalgae in the area (Dailer et al. 2012). In temperate and tropical regions, increasing eutrophication can lead to the accumulation of biomass of opportunistic macroalgae of Chlorophyta with simple morphologies, including
Bloom formation of
Other alternatives to the elucidation of this problem involving the bloom in the city of Garopaba may be related to the operation of currents, local oceanographic conditions, and / or decline in populations of predators. No related studies regarding the first two parameters have been found. In the case of predation, different studies suggest that the productivity of the system and higher trophic level consumers can jointly control the production of algae, suggesting that the effect of nutrients on the growth of algae blooms also depends on the top-down force (Worm et al. 2002).
The destination of algae biomass is a problem for coastal communities. They are likely to thrown out frequently. However, it can also have intrinsic economic value (Carmichael et al. 2000, Gupta et al. 2012). In the case of the Garopaba bloom, the dominant species
In summary, the marine macroalgal bloom on Garopaba Center Beach of Santa Catarina consisted of twentyfour algal taxa. It also included three species of bryozoans. This bloom was considered to be composed of heterogeneous biomass strains. This is the first time that