Amphibians are good “bioindicator species” that reflect environmental changes (Hopkins 2007, Lunde and Johnson 2012). Their morphological and behavioural characteristics and inhabiting under/near water with permeable skin during all life-cycle stages make them ideal organisms to assess local environmental health, particularly that of aquatic environments. Accumulating evidence suggests a decline in amphibian populations in many areas worldwide which apparently is attributed to multiple causes (Houlahan et al. 2000, Blaustein and Johnson 2003, Ohmer and Bishop 2011). A number of reasons have been suggested for this decline, including loss of habitat, environmental contaminants, pathogen outbreaks, and diseases (Kiesecker et al. 2001, Blaustein and Johnson 2003).
Herpetological studies have also continuously reported the incidences of abnormally developed body parts in anuran populations (Hebard and Brunson 1963, Lunde and Johnson 2012). Many different abnormalities have been documented, such as digit/limb malformation and missing or extra digits/limbs (Reeves et al. 2013, Lunde and Johnson 2012). Environmental changes, either due to climate change or human activities, such as UV-B radiation, chemical contaminants, or climate-change-induced emergence of pathogens (Skerratt et al. 2007, Lunde and Johnson 2012), are conceivably the main drivers for the emergence of abnormally developed amphibians.
These abnormalities have been reported primarily in amphibians inhabiting only in lentic habitats, such as ponds or lakes (Johnson et al. 2001, Reeves et al. 2013), whereas abnormalities in lotic-inhabiting amphibians are scarcely reported, either due to rarity or because it may be difficult to survey a sufficient number of frogs to quantify abnormalities in these environments (Lunde and Johnson 2012).
>
Study species and field sites
Based on the Daum Map (Daum Kakao 2015), a web-based map service used to visualize South Korean geography, we mapped out a location within which potential
>
Quantifying the degree of human land use
We developed two variables to adequately estimate the level of human activity at each site, such as the minimum distance between a focal site and the location of the nearest land used by humans and the proportion of human land use within a 300-m radius. Because our surveyed locations were forested land, the human land use was defined as non-forested area in the map, and by using the map, the human land use was easily distinguished. The 300-m criterion was specified because it is considerably less than the mean migration distances of
We first specified the area with a 300-m radius at each sampling site. Then we took a screenshot of the 300-m radius area and saved it as a JPEG file. Next, we selected the land area used by humans using the ‘polygon selection tool’ in ImageJ 1.46r (open source program, National Institutes of Health, Bethesda, MD, USA) and calculated the proportion of land used by humans. We also recorded the type of human activity close to the study site, which varied significantly among locations. Therefore, we did not use this category for statistical analysis but provide it in the Appendix.
We used ordinary least square regression to determine the relationship between the rate of abnormalities and two predictor variables, such as the proportion of land used by humans and the minimum distance between a site and the closest land used by humans. The proportion of land used by humans was normally distributed. However, we log transformed the minimum distance between frog habitat and the closest land used by humans to meet the assumption of ordinary least squares regression. As the two predictor variables of human activity were correlated each other (Pearson’s product moment correlation;
Additionally, we employed a nonparametric method for the frog abnormalities to avoid a large datum effect on the proportion data because of the low sample size at each site. All predictor and response variables were binary coded. Abnormality was zero, if no abnormal individual was found, or one, if any abnormal individual was found. Distance was 1 if a study site was < 100 m from human land use, and 0, if it was ≥ 100 m. The proportion was 1 if the proportion of human land use was ≥ 1% of the 300-m radius area, or 0, if it was < 1%. These criteria were chosen because it clearly distinguished the area with human activity and without it. The location with less than 1% of human land use indicates that there were hardly any human activities near the region.
Then, we used Fisher’s exact test to identify the association between human land use and the incidence of abnormal frogs.
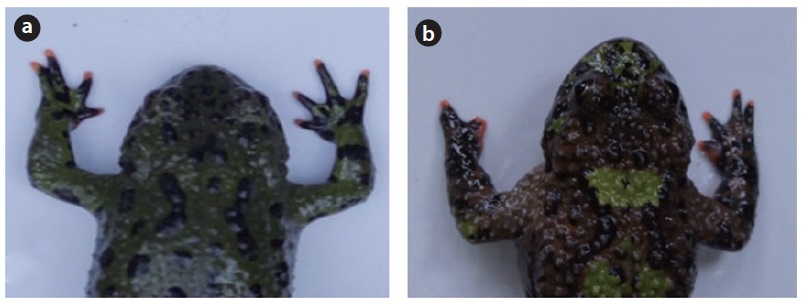
We found 228 frogs at 13 sites, but only 213 frogs (61 females, 151 males, and 1 unknown) that were collected from 10 sites were used for the analysis after excluding 3 sites with sample sizes < 15. Among the 213 individuals, 19 showed abnormal body parts, of which 18 had the malformed (Fig. 1a) or missing digits (Fig. 1b), and one individual had extra digits.
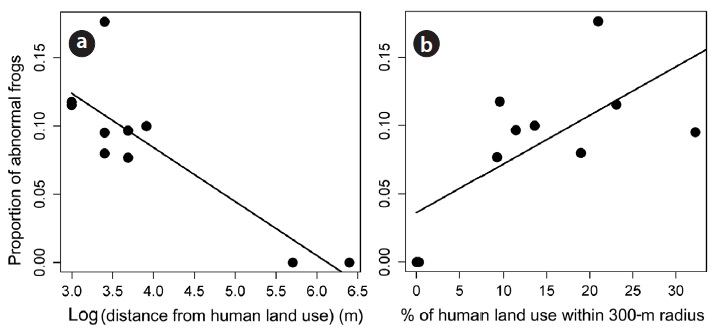
We found a significant relationship between the human land use and the abnormality rate in adult
In accordance with many other studies that have addressed the impact of human activities on frog malformations, our results suggest that the extent of human land use can increase the number of abnormalities in frogs and can negatively impact amphibian conservation in lotic environments. Noticeably, we always found abnormal individuals at sampling study sites where human activity was within a 100-m radius, regardless of the type of human land use (see Appendix for human land use types), but we found no abnormal frogs when human land use was located > 300-m radius from a frog habitat.
The reasons for the observed abnormalities are unknown, and interactions between several factors may have contributed to the observed abnormalities (Blaustein and Johnson 2003). We argue that human-generated chemicals and effluent water from commercial and residential facilities are the main drivers of the abnormalities. UV-B radiation is unlikely the reason for the observed abnormality because i)
Alternatively, anthropogenic chemicals may be responsible for abnormalities in
We note here that the abnormality rates in our study should be considerably lower than true abnormality rates in nature. First, because our survey started in August, which is after most
To the best our knowledge, this is the first attempt to address amphibian abnormalities in a lotic environment as related to human activity levels. These kinds of studies are important because they reflect ecological health, which will consequently affect human health. In line with the previous studies (Kiesecker et al. 2001, Taylor et al. 2005, Hopkins 2007, Mann et al. 2009), our results further support the idea that human activities negatively impact amphibian conservation. Our finding that the sites with frog abnormalities were in close proximity to land used by humans suggests that ecological health can be retained/improved by maintaining human land use away from the area of interest (Taylor et al. 2005, Reeves et al. 2008). It is desirable to incorporate a more detailed field survey and chemical analysis to firmly conclude the negative impact of human land use on amphibian abnormalities and the effects of how different types of land use. These studies will offer a future direction for amphibian conservation and to improve ecological health.