Inositol is an important nutrient present naturally in human milk and cow’s milk but in a lesser quantity. It exists predominantly in the free
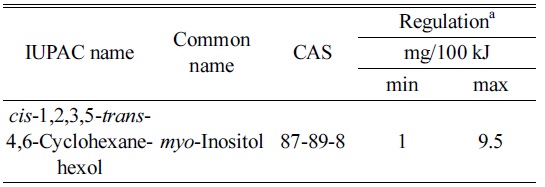
The levels of inositol in infant formula are regulated. CODEX1 defines lower and upper levels of inositol at 1 and 9.5 mg/100 kJ. In the USA there is no prescribed limits2 for inositol in dairy-based IF. The minimum amount of inositol in non-dairy based IF according to the Code of Federal Regulations (CFR) is 4 mg/100 kcal (~1 mg/100 kJ) and the upper limit is not regulated. The levels of inositol in IF in Australia and New Zealand are regulated by the FSANZ 2.9.1 and in China by the GB 10765-2010 Food Standards, Table 1.
[Table 1.] Chemical identity and regulatory limits of inositol
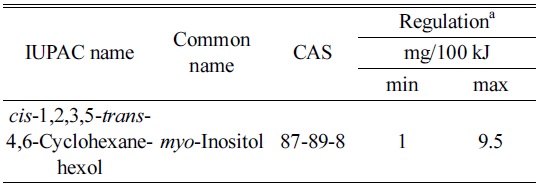
Chemical identity and regulatory limits of inositol
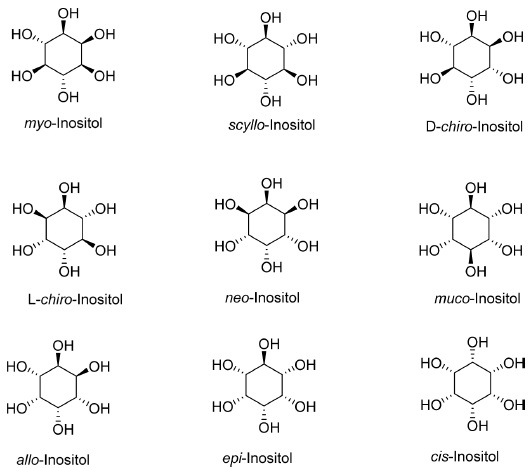
There are nine isomers of inositol C6H12O6 with the
There are several testing methods for inositol - GC/FID4,5 LC6,7,8,9 anion exchange chromatography10 and microbiological.11,12 The performance requirements set by the Association of Analytical Communities (AOAC International) with respect to inositol methods describe the determination of the free
All reagents were of analytical grade unless otherwise specified. Solvents and reagents for UPLC mobile phases were of
The
Dairy-based IF dry powder products were purchased from different retail outlets of the metropolitan Melbourne during June-August 2014. Before analysis samples were stored according to the label requirements. Free and bound inositols were extracted according to AOAC official methods 2012.1213 and bound inositols were hydrolyzed to the free form according to 2011.18.14 Both AOAC protocols were followed precisely in order to have consistency with data of other laboratories. Extracts were analyzed by UPLC-MS/MS.
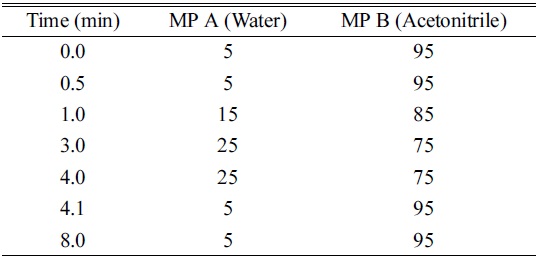
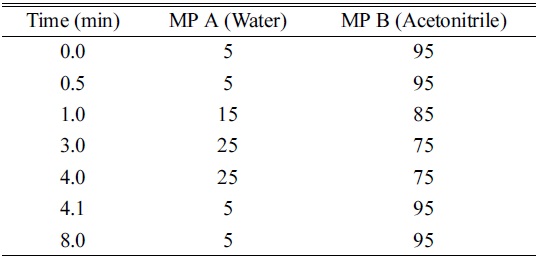
UPLC Gradient
Quality assurance and quality control was performed by analysis of reagent blanks, duplicate samples and SRM 1849a, NIST USA.
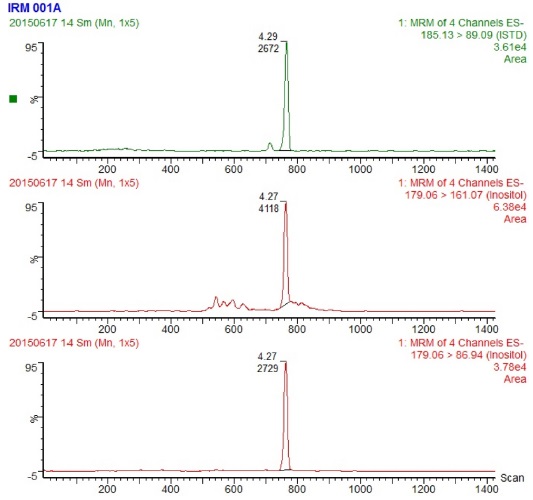
The extraction of free and bound inositol was based on official AOAC procedures, and the LCMS determination was developed and validated in-house based on publications of J.-H. Ahn15
Reference material, SRM1849a with stated
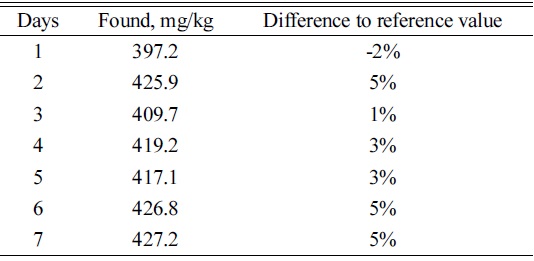
[Table 3.] Results of myo-inositol in SRM1849a analyzed on 7 separate occasions
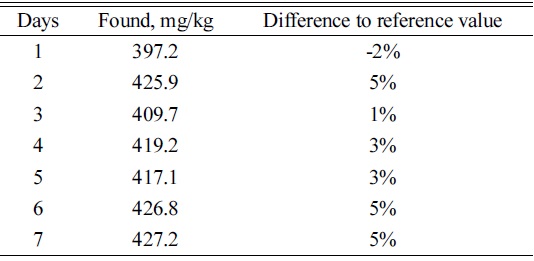
Results of myo-inositol in SRM1849a analyzed on 7 separate occasions
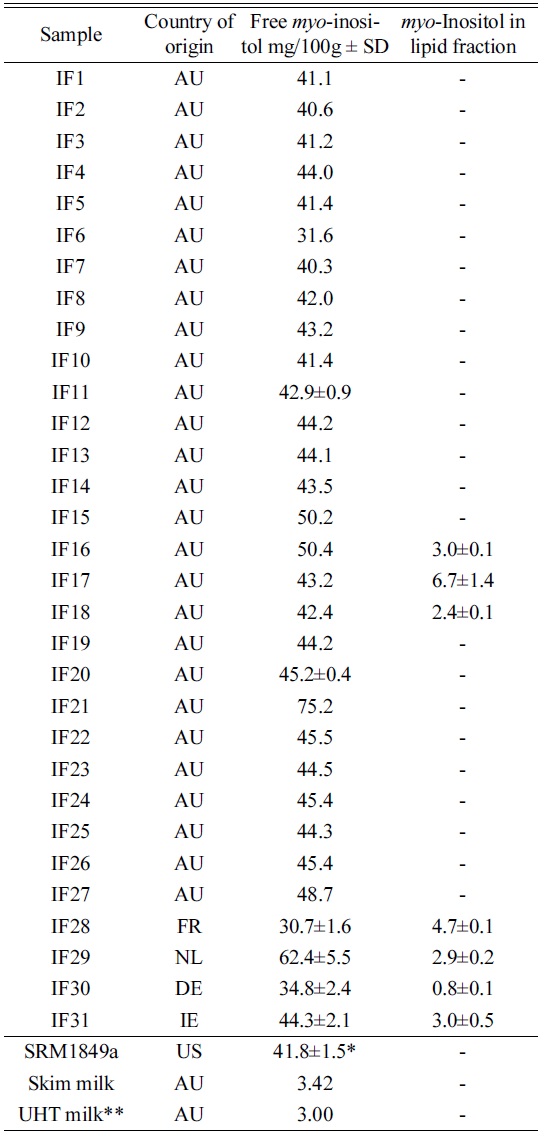
Table 4 shows the result of the survey. The results showed a mean value of 43.5 mg/100 g for Australian IF, excluding one outlier (IF21). Thermo17 recently published brief results and method. The microbiological and liquid chromatography methods of total inositol determination have been compared during analysis of dietetic milk powders. Several authors reported systematically higher concentrations of total inositol determined by the LC/PAD technique (420 to 1340 mg/kg) than by the microbiological procedure (270 to 1120 mg/kg). The difference between values given by two methods is significant considering mild extraction conditions applied for free inositol determination of both methods. To the contrary, Indyk and Woollard reported18 similar amounts of free inositol in skimmed milk powders, whole-milk powders and milk infant formulas determined by HPLC and microbiological procedures. Soy beans are known to contain up to 0.9 % of pinitol, a methylated form of
[Table 4.] Content of inositol in IF samples, SD is indicated for n=2.
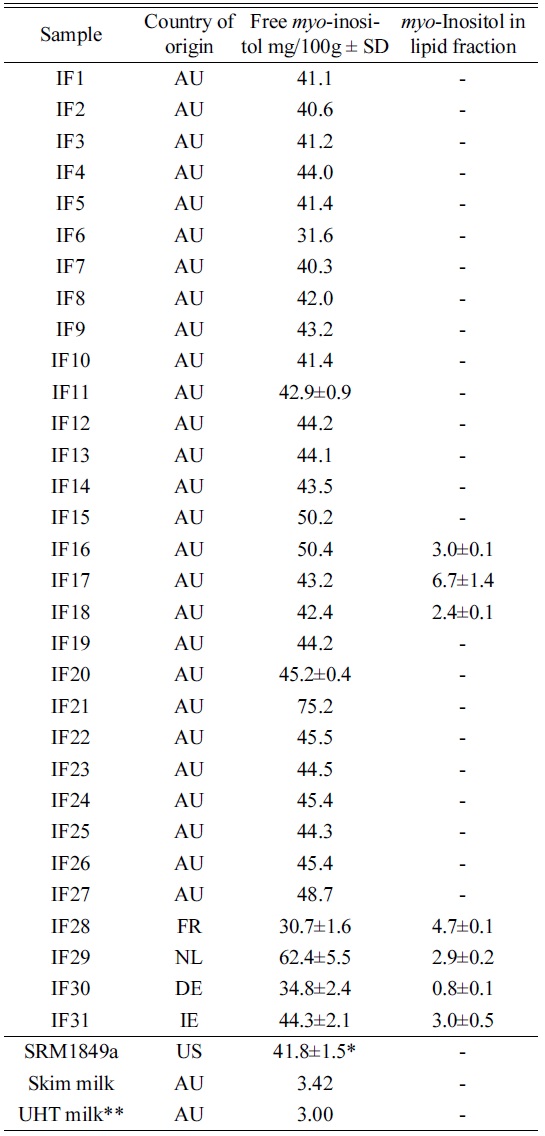
Content of inositol in IF samples, SD is indicated for n=2.
Following the protocols of NATA’s Technical Note 33,21 the MU for
A UPLC-MS/MS method was developed for the analysis of free